Performance of a real model: deconvolution of colorectal cancer samples
Source:vignettes/realModelExample.Rmd
realModelExample.RmdIn Building new deconvolution models was shown the workflow to build new deconvolution models. However, a ‘toy’ example was used in order to avoid long running times. In this vignette, the performance of a real model is shown in order to serve as a guide on how to assess whether a model has been correctly trained and can be used to deconvolute new bulk RNA-Seq samples.
Loading and inspection of the model
The model to be shown has been built with data from Li et al. (2017) (GSE81861), so it can be used to deconvolute samples from colorectal cancer. It is loaded from digitalDLSorteRdata package and the corresponding trained model can be used through the digitalDLSorteRmodels package.
suppressMessages(library(ggplot2))
suppressMessages(library(digitalDLSorteR))
if (!requireNamespace("digitalDLSorteRdata", quietly = TRUE)) {
install.packages(
"digitalDLSorteRdata",
repos = "https://diegommcc.github.io/digitalDLSorteRdataRepo/"
)
}
suppressMessages(library(digitalDLSorteRdata))
data(DDLSLiComp.list)
DDLSLiComp <- listToDDLS(DDLSLiComp.list)
DDLSLiComp## An object of class DigitalDLSorter
## Real single-cell profiles:
## 0 features and 0 cells
## rownames: ---
## colnames: ---
## Cell type composition matrices:
## Cell type matrix for traindata: 13334 bulk samples and 10 cell types
## Cell type matrix for testdata: 6666 bulk samples and 10 cell types
## Trained model: 30 epochs
## Training metrics (last epoch):
## loss: 0.0376
## accuracy: 0.9265
## mean_absolute_error: 0.0117
## categorical_accuracy: 0.9265
## Evaluation metrics on test data:
## loss: 0.0614
## accuracy: 0.9297
## mean_absolute_error: 0.0141
## categorical_accuracy: 0.9297
## Performance evaluation over each sample: MAE MSE
## Project: DigitalDLSorterProjectAs shown above, the model was trained 30 epochs with a total of
20,000 pseudo-bulk samples (13,334 for training and 6,666 for testing).
Now, evaluation metrics will be calculated by the
calculateEvalMetrics function in test data to explore its
performance in depth.
DDLSLiComp <- calculateEvalMetrics(DDLSLiComp)How errors are distributed
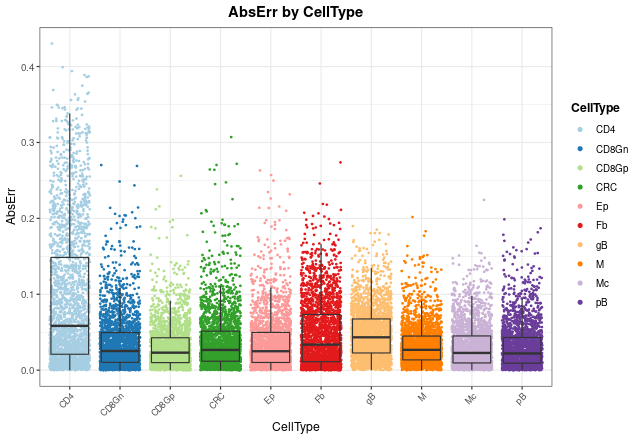
With distErrorPlot, we can plot in different ways how
errors are distributed. In this case, absolute error
(AbsErr) by cell type (CellType) is shown. CD4
T cell proportions are predicted worse than the other cell types, but
still have a median error close to zero.
distErrorPlot(
DDLSLiComp,
error = "AbsErr",
x.by = "CellType",
color.by = "CellType",
error.labels = FALSE,
type = "boxplot",
size.point = 0.5
)
In order to see mean error values, we can check it with
barErrorPlot:
barErrorPlot(DDLSLiComp, error = "MAE", by = "CellType")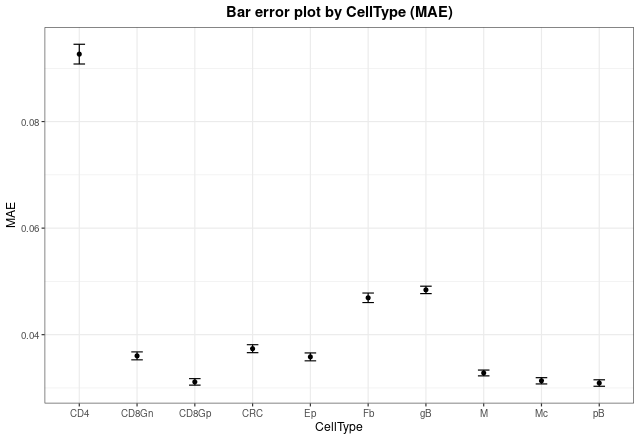
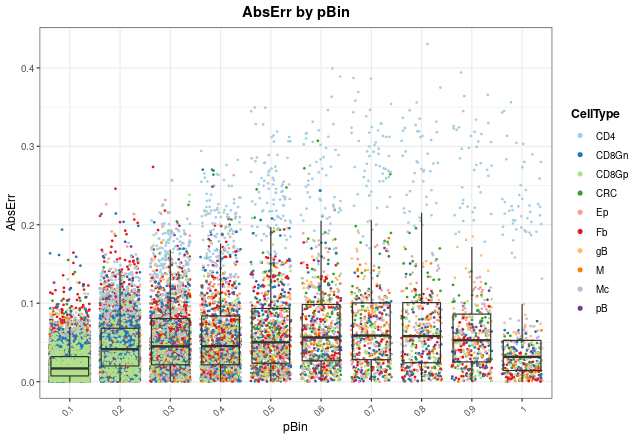
Now, we can see in which proportions the model is failing most by
changing the x.by argument to "pBin" and
optionally setting facet.by = "CellType":
distErrorPlot(
DDLSLiComp,
x.by = "pBin",
error = "AbsErr",
color.by = "CellType",
type = "boxplot",
size.point = 0.5
)
distErrorPlot(
DDLSLiComp,
x.by = "pBin",
error = "AbsErr",
facet.by = "CellType",
color.by = "CellType",
error.label = TRUE,
type = "boxplot"
)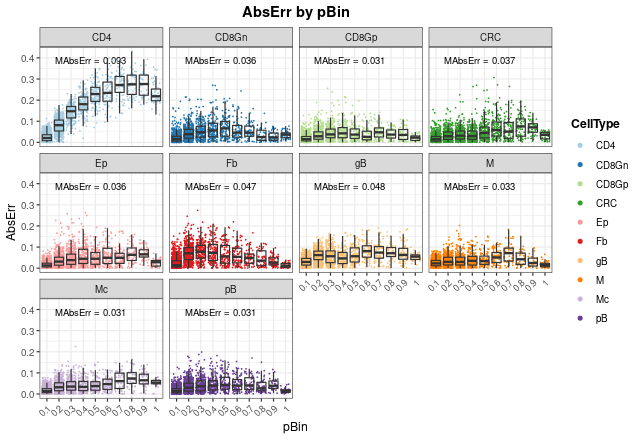
As can be seen, most of the failures in CD4 T cell proportions occur
at high proportions (between 0.5 and 1), and mean absolute errors
(MAbsErr) shown as annotations in each panel are very low
in all cases.
Correlation between actual and expected proportions
Another way to visualize how the model works is to use the
corrExpPredPlot function. Like distErrorPlot,
we can see how proportions are correlated with each other by different
variables. Here, we can see the same trend observed above: the model
performs worst estimating CD4+ T cell proportions with the lowest
concordance correlation and Pearson’s coefficients (\(R = 0.95\) and \(CCC = 0.794\), respectively). In fact, we
can observe that the model tends to underestimate these proportions,
although these values are still good. For the rest of cell types, the
results show a very good performance with high coefficients (between
0.97 and 0.99).
corrExpPredPlot(
DDLSLiComp,
color.by = "CellType",
facet.by = "CellType",
corr = "both",
size.point = 0.5
)## `geom_smooth()` using formula 'y ~ x'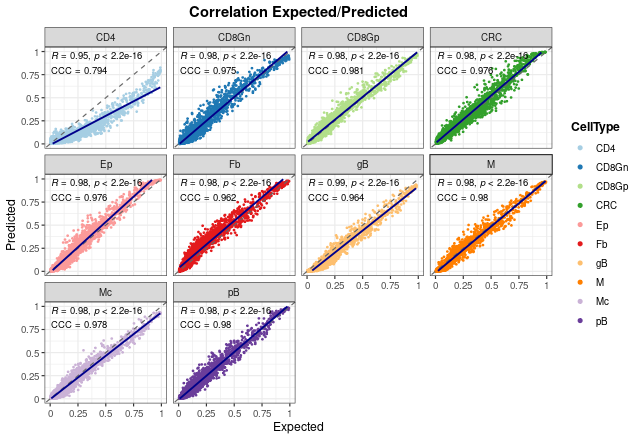
It is important to note that these results only show the estimated proportions of pseudo-bulk samples. If we don’t filter out single-cell profiles, the results improve in general, showing that the model is able to deal with pure samples as well.
corrExpPredPlot(
DDLSLiComp,
color.by = "CellType",
facet.by = "CellType",
size.point = 0.5,
filter.sc = FALSE,
corr = "both"
)## `geom_smooth()` using formula 'y ~ x'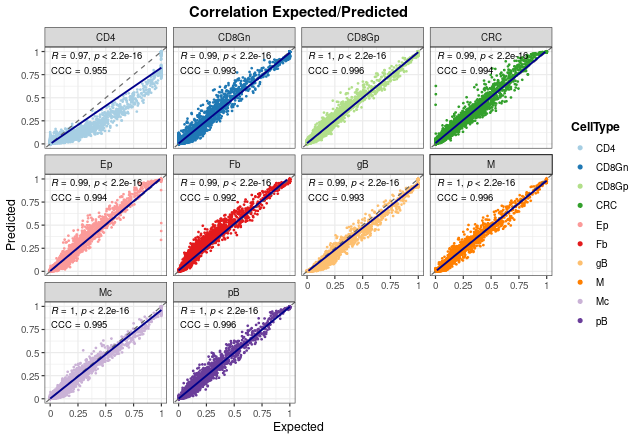
In the end, we can see the overall results without splitting the graph. As can be seen, both coefficients present also high values when all proportions are considered.
corrExpPredPlot(
DDLSLiComp,
color.by = "CellType",
size.point = 0.5,
corr = "both"
)## `geom_smooth()` using formula 'y ~ x'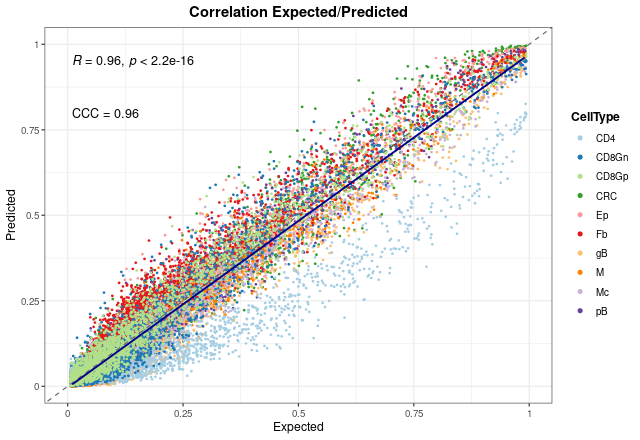
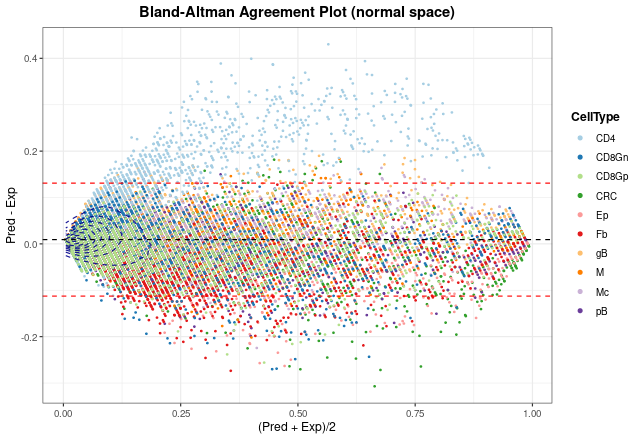
Bland-Altman agreement plots
The last way to graphically represent results is to use
blandAltmanLehPlot. It generates a Bland-Altman agreement
plot, a method for analyzing the level of agreement between two
variables, in that case expected vs predicted cell proportions. As
shown, most of the proportions fall close to zero (note blue density
lines) and the dashed red lines are very close to the mean, although we
can observe again the aforementioned problem in the estimate of T CD4
cell proportions.
blandAltmanLehPlot(
DDLSLiComp,
color.by = "CellType",
size.point = 0.5,
filter.sc = TRUE,
density = TRUE
)
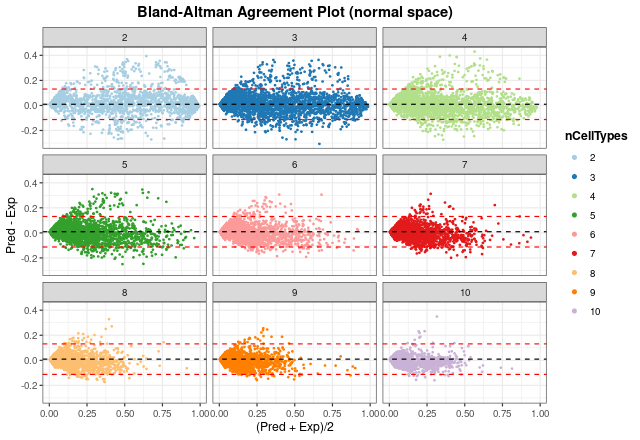
Finally, we can generate the same plot but spliting the information
by number of different cell types present in each pseudo-bulk sample.
The smaller the number of cell types, the larger the error made by the
model. In any case, considering the results from
distErrorPlot and corrExpPredPlot, we can
conclude that the model performs well on test data.
blandAltmanLehPlot(
DDLSLiComp,
color.by = "nCellTypes",
facet.by = "nCellTypes",
log.2 = FALSE,
size.point = 0.5,
filter.sc = TRUE,
density = FALSE
)
digitalDLSorteR dealing with real data from TCGA project
Now, to show the performance of this model on real data, colorectal cancer samples from The Cancer Genome Atlas (TCGA) project (Koboldt et al. 2012; Ciriello et al. 2015) will be used. This dataset consists of 521 samples and includes different subtypes of tumors and healthy samples.
data(TCGA.colon.se)
TCGA.colon.se## class: SummarizedExperiment
## dim: 59085 521
## metadata(0):
## assays(1): counts
## rownames(59085): 5S_RRNA 7SK ... ZZEF1 ZZZ3
## rowData names(0):
## colnames(521): X0096f6b3.94f5.49d9.aa43.66d9ab6b2b5c X01f5a228.a2cf.444f.8102.a9d4a2d9a27d ...
## ff016e44.7a4d.4423.822e.aa6aa6df0ef2 ffbe7115.06be.4592.91d0.3dad62e3c73a
## colData names(2): Bulk Tumor_TypeAs shown in Building new deconvolution
models, we can load the data into the DigitalDLSorter
object as follows:
DDLSLiComp <- loadDeconvData(
object = DDLSLiComp,
data = TCGA.colon.se,
name.data = "TCGA.colon"
)Then, with the deconvDigitalDLSorterObj function, these
new samples can be deconvoluted into the cell types considered by the
model and predicted proportions can be represented by the
barPlotCellTypes function.
DDLSLiComp <- deconvDigitalDLSorterObj(
object = DDLSLiComp,
name.data = "TCGA.colon",
verbose = FALSE
)
barPlotCellTypes(DDLSLiComp, name.data = "TCGA.colon", rm.x.text = TRUE)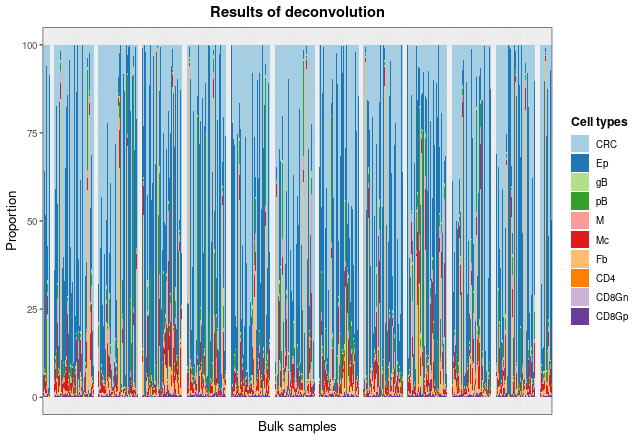
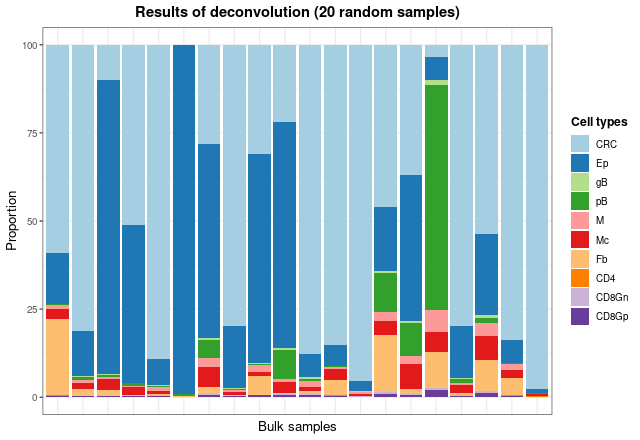
As the total number of samples is too high, we can see the results of
some samples by taking the matrix with the proportions and plotting 20
random samples with barPlotCellTypes:
set.seed(12345)
resDeconvTCGA <- deconv.results(DDLSLiComp, "TCGA.colon")
barPlotCellTypes(
resDeconvTCGA[sample(1:521, size = 20), ], rm.x.text = TRUE,
title = "Results of deconvolution (20 random samples)"
)
Finally, to check whether predicted estimates make sense with the type of sample they come from (metastatic, normal, primary, or recurrent), we will inspect predicted proportions of tumor cells, epithelial cells and fibroblasts.
dfProp <- data.frame(
Sample = rownames(resDeconvTCGA),
CRC = resDeconvTCGA[, "CRC"],
Ep = resDeconvTCGA[, "Ep"],
Fb = resDeconvTCGA[, "Fb"],
TypeSample = TCGA.colon.se@colData$Tumor_Type
)
dfProp <- reshape2::melt(dfProp)## Using Sample, TypeSample as id variables
ggplot(dfProp, aes(x = TypeSample, y = value, fill = TypeSample)) +
geom_boxplot() + facet_wrap(~ variable) + ylab("Estimated proportion") +
ggtitle("Estimated proportions in TCGA data") + theme_bw() +
theme(
plot.title = element_text(face = "bold", hjust = 0.5),
legend.title = element_text(face = "bold")
)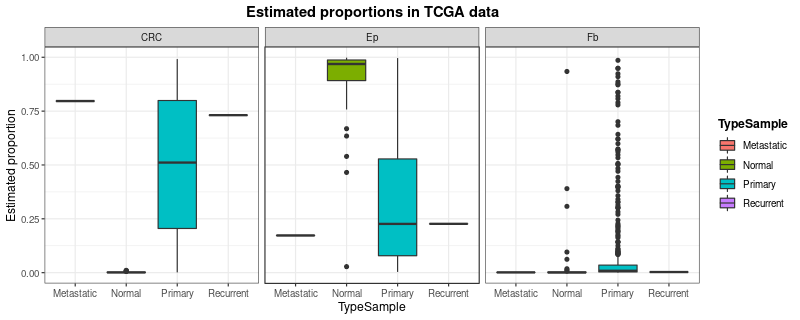
digitalDLSorteR correctly estimates the proportion
of tumor cells (CRC) and epithelial/fibroblast cells
(Ep and Fb) according to sample type. Further
analysis can be performed to better understand and evaluate the
performance of the model on these data (correlation analysis between
estimated cell proportions, comparisons with other published tools,
etc.).